Interesting, and depressing, statistics from the Online Education Database titled 8 Astonishing Stats on Academic Cheating.
1. 60.8% of polled college students admitted to cheating.
2. The same poll revealed that 16.5% of them didn't regret it.
3. Cheaters have higher GPAs.
4. The public is more concerned with cheating than college officials.
5. Cheating college students likely start in high school.
6. In fact, 85% of them think cheating is essential.
7. 95% of cheaters don't get caught.
8. Top-tier paper mill website average about 8,000 hits a day.
Go read it for more details on each point.
The article does say that math and science classes inspire the most incidents which obviously interests me. Whenever I require a paper now, I require students to submit it to Turnitin first to check for plagiarism - students still try to get away with it, just a couple of weeks ago a student handed in a paper with over 50% directly-copied content (no quotations, no citations).
I'm not naive, I know students cheat and plagiarize each other on assignments and labs. I encourage students to work together, but some take that to mean they can copy off their classmates. I generally know who's doing it (although I can't always prove it) and I always announce to the class that they're not going to be able to work together on the lab exam. And, predictably, a certain number of people fail the lab exam (the same ones I suspect are copying on labs all semester). Since the lab exam is worth a lot more than individual labs, they're only hurting themselves in the end.
I really do hate having to treat all students as potential cheaters. I'd rather be grading or reading something while students are taking exams instead of pacing around watching them like hawks. Unfortunately, past experience has shown that some people do try to cheat and that's not fair to the honest students.
I also surf the web looking at web sites on how to cheat. I'm wise to many of the tricks used!
Friday, December 31, 2010
Thursday, December 30, 2010
One year of blogging
I started this blog one year ago with the first post on December 29, 2009. It started off slow and I really wondered if I'd be able to keep it up. I surprised myself.
This is the 278th post, I've had almost 14,000 unique visitors, and I'm sometimes getting over 100 unique visitors a day. Not bad for a blog I haven't advertised much and people generally just stumble upon.
I enjoy writing and it's not usually a chore (sometimes, but not usually). Not sure if anyone enjoys reading any particular post but I do it for my own enjoyment (I can't explain why I enjoy writing, but I do).
I think I'll keep it up for 2011!
This is the 278th post, I've had almost 14,000 unique visitors, and I'm sometimes getting over 100 unique visitors a day. Not bad for a blog I haven't advertised much and people generally just stumble upon.
I enjoy writing and it's not usually a chore (sometimes, but not usually). Not sure if anyone enjoys reading any particular post but I do it for my own enjoyment (I can't explain why I enjoy writing, but I do).
I think I'll keep it up for 2011!
Bronze
Yesterday I discussed tin and its major ore mineral cassiterite (SnO2).
Tin is useful because it can be alloyed with copper to create bronze - typically 88% copper (Cu) and 12% tin (Sn) although ancient bronze pieces can vary in composition. The word is of uncertain origin but this metal alloy was historically important enough that we have a period of human history called the Bronze Age.
The earliest period of Homo sapiens history is called the Lithic or Stone Age (subdivided into the Paleolithic, Mesolithic, and Neolithic). During this time, humans made tools and weapons from stone - often flaked quartzite, chert (flint), or obsidian which chip into nice sharp edges.
One of the first metals to be used for tools and weapons was copper. Native copper (pure copper metal deposits) occur in a number of areas and copper is relatively soft and easy to work. The use of copper appears earliest in Asia and appears to quickly spread into Europe. In Serbia, a copper axe was found dating to 5300 BC. Ötzi the Iceman, found frozen in the Alps in 1991, had a copper axe and was dated to 3300 BC. Copper working independently arose in the New World appearing in the upper Midwest (from copper deposits in Michigan and Minnesota) and among the Inca in Peru.
The period of time when humans made copper tools is sometimes referred to as the Chalcolithic Age (from the Greek χαλκός for copper). Relatively quickly, somewhere in the Fertile Crescent, it was discovered that if a little tin was added to copper, it made a much harder metal. This occurred around 3500 BC. This was the result of intentional experimentation since copper and tin don't normally occur together as mineral ores. Tin has to be mined and smelted separately (from the mineral cassiterite), and then added to copper to obtain bronze.
Copper is available throughout Europe and Asia, but tin is a bit less common. Starting in the early Bronze Age, tin was mined from the Cornwall and Devon areas of southwest England and was eventually traded throughout Europe. Much of the inter-cultural communication throughout Europe and Asia in ancient times was a result of mineral resource trading.
Tin is useful because it can be alloyed with copper to create bronze - typically 88% copper (Cu) and 12% tin (Sn) although ancient bronze pieces can vary in composition. The word is of uncertain origin but this metal alloy was historically important enough that we have a period of human history called the Bronze Age.
The earliest period of Homo sapiens history is called the Lithic or Stone Age (subdivided into the Paleolithic, Mesolithic, and Neolithic). During this time, humans made tools and weapons from stone - often flaked quartzite, chert (flint), or obsidian which chip into nice sharp edges.
One of the first metals to be used for tools and weapons was copper. Native copper (pure copper metal deposits) occur in a number of areas and copper is relatively soft and easy to work. The use of copper appears earliest in Asia and appears to quickly spread into Europe. In Serbia, a copper axe was found dating to 5300 BC. Ötzi the Iceman, found frozen in the Alps in 1991, had a copper axe and was dated to 3300 BC. Copper working independently arose in the New World appearing in the upper Midwest (from copper deposits in Michigan and Minnesota) and among the Inca in Peru.
Sumerian copper dagger (c. 2500 BC)
The period of time when humans made copper tools is sometimes referred to as the Chalcolithic Age (from the Greek χαλκός for copper). Relatively quickly, somewhere in the Fertile Crescent, it was discovered that if a little tin was added to copper, it made a much harder metal. This occurred around 3500 BC. This was the result of intentional experimentation since copper and tin don't normally occur together as mineral ores. Tin has to be mined and smelted separately (from the mineral cassiterite), and then added to copper to obtain bronze.
Bronze weapons from Canaan (c. 2000 BC)
Copper is available throughout Europe and Asia, but tin is a bit less common. Starting in the early Bronze Age, tin was mined from the Cornwall and Devon areas of southwest England and was eventually traded throughout Europe. Much of the inter-cultural communication throughout Europe and Asia in ancient times was a result of mineral resource trading.
Tuesday, December 28, 2010
Tin
Tin is a metallic element (number 50 on the periodic table) and abbreviated Sn. The word tin is of Germanic origin and the Sn for the symbol comes from its Latin name stannum. Tin is directly above lead (Pb) on the periodic table with which it shares some chemical properties.
Tin has two oxidation states, +2 and +4 with the +4 being more stable. This leads to a common oxide ore mineral of tin, cassiterite, with the formula SnO2 (oxygen has a -2 oxidation so the tin is +4 in cassiterite). The name derives from the Greek kassiteros (tin) derived from the Phoenician word for Britain (Cassiterid), an important source of tin in the ancient world (a topic we'll return to tomorrow).
 Placer mining was very important in the state of Perak in northwestern peninsular Malaysia near the city of Ipoh (see map). In a very labor-intensive practice, pits were dug into alluvial gravels and the sediments were then washed over boards with riffle traps catching the heavy cassiterite grains (similar to the way placer gold mining is done, see diagram at left as a simple example).
Placer mining was very important in the state of Perak in northwestern peninsular Malaysia near the city of Ipoh (see map). In a very labor-intensive practice, pits were dug into alluvial gravels and the sediments were then washed over boards with riffle traps catching the heavy cassiterite grains (similar to the way placer gold mining is done, see diagram at left as a simple example).
Tin has two oxidation states, +2 and +4 with the +4 being more stable. This leads to a common oxide ore mineral of tin, cassiterite, with the formula SnO2 (oxygen has a -2 oxidation so the tin is +4 in cassiterite). The name derives from the Greek kassiteros (tin) derived from the Phoenician word for Britain (Cassiterid), an important source of tin in the ancient world (a topic we'll return to tomorrow).
Cassiterite has a high density and its specific gravity, around 7.0, means its 7 times heavier than an equal volume of water. This leads to cassiterite occuring as placer deposits in many areas. The cassiterite weathers out of rocks and is concentrated by streams into alluvial placer deposits much like gold.
 Placer mining was very important in the state of Perak in northwestern peninsular Malaysia near the city of Ipoh (see map). In a very labor-intensive practice, pits were dug into alluvial gravels and the sediments were then washed over boards with riffle traps catching the heavy cassiterite grains (similar to the way placer gold mining is done, see diagram at left as a simple example).
Placer mining was very important in the state of Perak in northwestern peninsular Malaysia near the city of Ipoh (see map). In a very labor-intensive practice, pits were dug into alluvial gravels and the sediments were then washed over boards with riffle traps catching the heavy cassiterite grains (similar to the way placer gold mining is done, see diagram at left as a simple example).Malaysian tin mining began in the 1840's, provided over 30% of the world's tin around 1980, but is only a minor source today since the price of tin on the world market is too low to make these mines economical.
Tin is not mined in the United States. Most tin today comes from China and Indonesia. In China, deposits occur in an area called the Southeast Yunnan Tin Belt. The Yunnan Province in on China's southern boundary and the tin belt is south of the provincial capital of Kunming and just north of China's border with Vietnam (see map). Tin here occurs in quartz veins associated with Mesozoic-aged granitic intrusions. In Indonesia, tin is mined on Bangka Island ("Tin Island") off the southeastern coast of Sumatra (see map). Much of the mining here are alluvial placer deposits derived from Mesozoic-aged intrusives as well.
Tin is not mined in the United States. Most tin today comes from China and Indonesia. In China, deposits occur in an area called the Southeast Yunnan Tin Belt. The Yunnan Province in on China's southern boundary and the tin belt is south of the provincial capital of Kunming and just north of China's border with Vietnam (see map). Tin here occurs in quartz veins associated with Mesozoic-aged granitic intrusions. In Indonesia, tin is mined on Bangka Island ("Tin Island") off the southeastern coast of Sumatra (see map). Much of the mining here are alluvial placer deposits derived from Mesozoic-aged intrusives as well.
Most tin produced today (over 50%) is used as an alloy in solders (metal pipe and electrical connections). Much of the rest is used in tin plating of various metals for corrosion resistance and in various chemicals. Tin was formerly used a lot more in the production of tin cans and tin foil but has now been replaced by aluminum.
Tin is historically interesting because it can be alloyed with copper to create bronze. I'll leave that for tomorrow's post.
Tin is historically interesting because it can be alloyed with copper to create bronze. I'll leave that for tomorrow's post.
Monday, December 27, 2010
Ozzy and Keith
I love to read. Most of what I read is non-fiction and a lot of it is in the genres of science/nature/travel. Occasionally, however, I'll read biographies and autobiographies and I recently read two that surprised me - both from infamous British rock stars.
Keith Richards Life (Little, Brown, & Company, 2010) reads like much of it was transcribed from recordings and could have benefited from some editing to tighten it up (the book is over 500 pages). Many of the stories are simply recitations of what he did when which I found boring to read after a while.
(Little, Brown, & Company, 2010) reads like much of it was transcribed from recordings and could have benefited from some editing to tighten it up (the book is over 500 pages). Many of the stories are simply recitations of what he did when which I found boring to read after a while.
The thing that surprised me the most, not having read much about the Rolling Stones in the past, was how Keith Richards is a complete narcissistic jerk. From the decades of self-indulgent drug use, to criminal child neglect of his son Marlon (who he crashed a car into a tree with when wasted and who he took on tour), to his misogynistic treatment of women, to leaving audiences waiting an hour or more whil he sleeps off his latest drug cocktail, to cheating on his wives, to threatening people with knives who dare criticize him, to an apparent complete lack or sorrow or remorse for all the people he hurt in his life.
In Richard's world, the cops and customs inspectors are all assholes because they keep busting him for drugs and unregistered pistols (which he freely admits to having at the time), women are Kleenex, and his overblown sense of entitlement leads him to expect that a few phone calls will get him out of anything (mostly true - I'd be rotting in prison if I was arrested for the same things). He's a right git.
There were a few interesting and humorous things in the book but not really enough to justify wading through 500+ pages of drivel. As a frustrated guitar player (and "player" is too strong a word), it was interesting to read about the open G tuning which gives many of the Stones' songs their distinctive sound. Keith Richards has also played with a lot of great musicians and there were a few tidbits there. It was also pretty funny when he described his relationship with Jagger. Richards once saw a book by an author named Brenda Jagger and would refer to Mick Jagger to other band mates as Brenda (as in "Brenda's on the rag today") - apparently with Mick being in the room and not realizing they were talking about him. He also talked about having a myna bird as a pet once but had to get rid of it because it was always yelling and screeching at him ("It was like having Mick in a cage in my room", he said).
Can't say as I would recommend the book. It would only be of interest to die-hard fans and, quite frankly, I'm puzzled by a number of the good reviews of it on Amazon. No accounting for taste.
The other book was I am Ozzy by Ozzy Osbourne (Grand Central Publishing, 2010). I have to say, in contrast to Keith Richard's dreck, I enjoyed this book quite a bit. The book is very readable and humorous and Ozzy comes across as a likable guy even though he did a lot of bad things in his life. He came a long way from the dyslexic kid who always got in trouble from a lower-class working family in Birmingham, England.
by Ozzy Osbourne (Grand Central Publishing, 2010). I have to say, in contrast to Keith Richard's dreck, I enjoyed this book quite a bit. The book is very readable and humorous and Ozzy comes across as a likable guy even though he did a lot of bad things in his life. He came a long way from the dyslexic kid who always got in trouble from a lower-class working family in Birmingham, England.
Like Richards, Ozzy had his troubles with drugs and alcohol but claims to be clean now. It's clear he is sorry for the people he hurt (especially his wife Sharon) and does have remorse for stupid things he's done in the past. Even though he did many of the same types of things Keith Richards did, he still comes across as a more decent character.
Lots of interesting and amusing stories about people and events. Ozzy going into a store the first time in America asking for 20 "fags" (slang for cigarettes in England) and the lady going "What do you want 20 fags for? Get the hell out of here you pervert!" Ozzy also had the reputation for being into satanism which was completely not true and he says it got real tiring dealing with people who took it all seriously (the guys from Black Sabbath wanted to write "scary" music). Once saw a group of satanists in a circle with candles at his hotel. Ozzy goes over to them, blows out the candles, and starts singing "Happy Birthday". Ozzy telling a doctor about all the drugs he did and the doctor turns to him and asks "Why are you still alive?" Sharon telling him to "Fuck off!" first time he proposed to her.
A good read if you're looking for something light and easy reading.
Keith Richards Life
The thing that surprised me the most, not having read much about the Rolling Stones in the past, was how Keith Richards is a complete narcissistic jerk. From the decades of self-indulgent drug use, to criminal child neglect of his son Marlon (who he crashed a car into a tree with when wasted and who he took on tour), to his misogynistic treatment of women, to leaving audiences waiting an hour or more whil he sleeps off his latest drug cocktail, to cheating on his wives, to threatening people with knives who dare criticize him, to an apparent complete lack or sorrow or remorse for all the people he hurt in his life.
In Richard's world, the cops and customs inspectors are all assholes because they keep busting him for drugs and unregistered pistols (which he freely admits to having at the time), women are Kleenex, and his overblown sense of entitlement leads him to expect that a few phone calls will get him out of anything (mostly true - I'd be rotting in prison if I was arrested for the same things). He's a right git.
There were a few interesting and humorous things in the book but not really enough to justify wading through 500+ pages of drivel. As a frustrated guitar player (and "player" is too strong a word), it was interesting to read about the open G tuning which gives many of the Stones' songs their distinctive sound. Keith Richards has also played with a lot of great musicians and there were a few tidbits there. It was also pretty funny when he described his relationship with Jagger. Richards once saw a book by an author named Brenda Jagger and would refer to Mick Jagger to other band mates as Brenda (as in "Brenda's on the rag today") - apparently with Mick being in the room and not realizing they were talking about him. He also talked about having a myna bird as a pet once but had to get rid of it because it was always yelling and screeching at him ("It was like having Mick in a cage in my room", he said).
Can't say as I would recommend the book. It would only be of interest to die-hard fans and, quite frankly, I'm puzzled by a number of the good reviews of it on Amazon. No accounting for taste.
The other book was I am Ozzy
Like Richards, Ozzy had his troubles with drugs and alcohol but claims to be clean now. It's clear he is sorry for the people he hurt (especially his wife Sharon) and does have remorse for stupid things he's done in the past. Even though he did many of the same types of things Keith Richards did, he still comes across as a more decent character.
Lots of interesting and amusing stories about people and events. Ozzy going into a store the first time in America asking for 20 "fags" (slang for cigarettes in England) and the lady going "What do you want 20 fags for? Get the hell out of here you pervert!" Ozzy also had the reputation for being into satanism which was completely not true and he says it got real tiring dealing with people who took it all seriously (the guys from Black Sabbath wanted to write "scary" music). Once saw a group of satanists in a circle with candles at his hotel. Ozzy goes over to them, blows out the candles, and starts singing "Happy Birthday". Ozzy telling a doctor about all the drugs he did and the doctor turns to him and asks "Why are you still alive?" Sharon telling him to "Fuck off!" first time he proposed to her.
A good read if you're looking for something light and easy reading.
Friday, December 24, 2010
Christmas and Yule
In Christianity, Christmas is the celebration of the birth of Jesus. Let's leave aside for now any discussion of the historicity of Jesus and simply assume the Biblical account of his birth is correct. Is there scriptural evidence that Jesus was born on December 25? The short answer is no. Shepherds weren't out in the fields in December. They were out March through October. If Jesus died at 33 1/2 years old, on Passover, his birthdate would have been in mid-September. A Google search will clearly reveal that a number of people (some better qualified to do so than others) argue rather strongly for one date or another, but no one seriously argues for December 25 (and he was likely born sometime around 2-5 BC as well).
Why do we celebrate on December 25? Because once the early Christian church became powerful enough, it sought to stomp out all the old pagan beliefs and mid-December was a time of feasting and celebration in ancient Europe. It's when the winter solstice occurs, the turning of the year, and the church couldn't have people engaged in Sun worship so, in 350, Pope Julius I declared that Christ’s birth would be celebrated on December 25. Many of the old pagan traditions were hard to stomp out, however, and carried over into our modern Christmas celebrations.
Holly, ivy, and mistletoe are all plants associated with pagan mid-winter celebrations. Mistletoe has an interesting cultural history. European mistletow grows as a semi-parastitic plant on oak trees (sacred to the druids) and remains as a green crown on the seasonally "dead" trees in winter. It was seen as a powerful fertility symbol. The waxy white berries were identified with drops of semen - think about that next time you kiss under the mistletoe. I've read that mistletoe was banned from early Christian churches due to its pagan associations.
Yule logs are another of the pagan holdovers (a Norse tradition). So are wreaths and cuttings of evergreens (Germanic). Pagans did not erect Christmas trees (probably because they wouldn't cut down a living tree and bring it into a house) but there is a long pagan history of decorating living trees. As an aside, some fundamentalist Christians refuse to have a Christmas tree in their home because of Jeremiah 10:1-5.
While the Santa Claus legend is usually attributed by Christians to St. Nicholas of Myra in the 4th century, Santa has a lot of parallels to the old Germanic/Norse legends of Odin. Around Yule, Odin would lead a great hunt riding an 8-legged horse named Sleipnir that could leap great distances (kind of like magical reindeer). Odin also was referred to as having a long beard. Children would place boots filled with carrots or straw near the fireplace for Sleipnir to eat and Odin would leave gifts in return (like hanging stockings by the fire).
I'm half Finn and in Finland Santa is called Joulupukki which literally means "Yule Goat". This likely comes from an old pagan tradition of evil spirits that wore goat skins and horns and came in the night to demand gifts. This later morphed into a more jolly figure. Joulupukki is where we get the reindeer, the red and white suit, the helper elves (Joulutonttu), and the cold North Pole origin (Lapland in Finland originally).
Bottom line is that what we call Christmas is a mish-mash of European traditions, both Christian and pagan. So, despite what you hear in church, Christ is not the only reason for the season (I think he'd be horrified by the greed and commercialism of the holiday anyway - see Matthew 21:12). The tilt of the Earth's axis in the European mid-latitude climate is the real reason we have a seasonal celebration - it's ultimately astronomical!
Merry Christmas!
Why do we celebrate on December 25? Because once the early Christian church became powerful enough, it sought to stomp out all the old pagan beliefs and mid-December was a time of feasting and celebration in ancient Europe. It's when the winter solstice occurs, the turning of the year, and the church couldn't have people engaged in Sun worship so, in 350, Pope Julius I declared that Christ’s birth would be celebrated on December 25. Many of the old pagan traditions were hard to stomp out, however, and carried over into our modern Christmas celebrations.
Holly, ivy, and mistletoe are all plants associated with pagan mid-winter celebrations. Mistletoe has an interesting cultural history. European mistletow grows as a semi-parastitic plant on oak trees (sacred to the druids) and remains as a green crown on the seasonally "dead" trees in winter. It was seen as a powerful fertility symbol. The waxy white berries were identified with drops of semen - think about that next time you kiss under the mistletoe. I've read that mistletoe was banned from early Christian churches due to its pagan associations.
Yule logs are another of the pagan holdovers (a Norse tradition). So are wreaths and cuttings of evergreens (Germanic). Pagans did not erect Christmas trees (probably because they wouldn't cut down a living tree and bring it into a house) but there is a long pagan history of decorating living trees. As an aside, some fundamentalist Christians refuse to have a Christmas tree in their home because of Jeremiah 10:1-5.
While the Santa Claus legend is usually attributed by Christians to St. Nicholas of Myra in the 4th century, Santa has a lot of parallels to the old Germanic/Norse legends of Odin. Around Yule, Odin would lead a great hunt riding an 8-legged horse named Sleipnir that could leap great distances (kind of like magical reindeer). Odin also was referred to as having a long beard. Children would place boots filled with carrots or straw near the fireplace for Sleipnir to eat and Odin would leave gifts in return (like hanging stockings by the fire).
I'm half Finn and in Finland Santa is called Joulupukki which literally means "Yule Goat". This likely comes from an old pagan tradition of evil spirits that wore goat skins and horns and came in the night to demand gifts. This later morphed into a more jolly figure. Joulupukki is where we get the reindeer, the red and white suit, the helper elves (Joulutonttu), and the cold North Pole origin (Lapland in Finland originally).
Bottom line is that what we call Christmas is a mish-mash of European traditions, both Christian and pagan. So, despite what you hear in church, Christ is not the only reason for the season (I think he'd be horrified by the greed and commercialism of the holiday anyway - see Matthew 21:12). The tilt of the Earth's axis in the European mid-latitude climate is the real reason we have a seasonal celebration - it's ultimately astronomical!
Merry Christmas!
Thursday, December 23, 2010
Monkeys in business suits
Oklahoma and Kansas are apparently battling it out to see who has the stupidest state legislators.
The Durant Daily Democrat newspaper (I had to look - Durant is a town in southern Oklahoma just over the border north of Dallas/Fort Worth, TX) published an opinion piece by recently-elected Oklahoma State Senator Josh Brecheen.
There are many other genuinely stupid things in Brecheen's article - see P.Z. Myers amusing comments at Phyrangula. There's no crime in being a moron. It is inadvisable, however, to write newspaper articles to put it on public record.
The Durant Daily Democrat newspaper (I had to look - Durant is a town in southern Oklahoma just over the border north of Dallas/Fort Worth, TX) published an opinion piece by recently-elected Oklahoma State Senator Josh Brecheen.
Here's his article: Brecheen discusses evolution and Darwinian Theory.
Looking past the bad grammar (the "its" vs. "it's" confusion really annoys me), he makes some amazingly stupid comments. Biologists would be very surprised to learn that if the theory of evolution were true, monkeys would be expected to climb out of trees and put on business suits (since we don't see that, Brecheen claims, evolution is false, QED). We also don't see insects turn into mammals - a bizarre idea the State Senator called "phyla cross-over" that's mysteriously been left out of biology textbooks.
I feel sorry for school kids in places like Ohlahoma or Kansas. They're in for quite the shock when they get to a real science class in college.
Looking past the bad grammar (the "its" vs. "it's" confusion really annoys me), he makes some amazingly stupid comments. Biologists would be very surprised to learn that if the theory of evolution were true, monkeys would be expected to climb out of trees and put on business suits (since we don't see that, Brecheen claims, evolution is false, QED). We also don't see insects turn into mammals - a bizarre idea the State Senator called "phyla cross-over" that's mysteriously been left out of biology textbooks.
I feel sorry for school kids in places like Ohlahoma or Kansas. They're in for quite the shock when they get to a real science class in college.
There are many other genuinely stupid things in Brecheen's article - see P.Z. Myers amusing comments at Phyrangula. There's no crime in being a moron. It is inadvisable, however, to write newspaper articles to put it on public record.
Wednesday, December 22, 2010
Busy couple of days
Didn't post yesterday but I've been busy.
Went to Atlantic City with the wife Monday night - not because we're gamblers (we're low rollers and spent about $25 in the slots before giving up) but because we can get a nice hotel room cheap ($44 at Ballys on the 40th floor with ocean view) and get some time away from our kids (who've been really annoying lately!).
After the 4+ hour drive back on Tuesday, I went out to sit up all night with some heathens around a fire to mark the Solstice and celebrate the return of the Sun rising over the Hudson River (very apropos after teaching Ancient Astronomy this semester). It's a connection to my Germanic pagan ancestry.
In between all of this, I had to read and grade final papers (some horribly written and one blatantly plagiarized) and calculate final grades. Oh yeah, our carbon monoxide detector in the basement went off and we had to have an emergency furnace cleaning. And our living room television went "pop" and is no more.
Like I said, busy couple of days.
Went to Atlantic City with the wife Monday night - not because we're gamblers (we're low rollers and spent about $25 in the slots before giving up) but because we can get a nice hotel room cheap ($44 at Ballys on the 40th floor with ocean view) and get some time away from our kids (who've been really annoying lately!).
After the 4+ hour drive back on Tuesday, I went out to sit up all night with some heathens around a fire to mark the Solstice and celebrate the return of the Sun rising over the Hudson River (very apropos after teaching Ancient Astronomy this semester). It's a connection to my Germanic pagan ancestry.
In between all of this, I had to read and grade final papers (some horribly written and one blatantly plagiarized) and calculate final grades. Oh yeah, our carbon monoxide detector in the basement went off and we had to have an emergency furnace cleaning. And our living room television went "pop" and is no more.
Like I said, busy couple of days.
Monday, December 20, 2010
This poll by Gallup has been done a number of times since 1982 (click to enlarge or click on the Gallup link).
Bottom line is that 4 in 10 Americans believe God created Adam and Eve in the Garden of Eden a few thousand years ago. Gallup doesn't ask, but I presume many, if not most of these respondents also believe in a young-Earth. In other words, they completely reject virtually all of modern science - once you throw a young-Earth in there, geology and astronomy are also out along with biology. In addition, to support a young-Earth creationist (YEC) model, chemistry and physics don't work either (e.g. the superfast plate tectonic model of some YECs or the "vapor canopy" model for flood waters is complete nonsense according to rather simple thermodynamics).
The way YECs explain things is much like the cartoon below.
I guess one could say there's good news in that 40% is the lowest it's been in decades. It is rather sad, however, that 4 in 10 Americans believe scientists are liars engaged in a global conspiracy. It's a good thing science is not decided by popular vote.
Sunday, December 19, 2010
Solstice eclipse
The winter solstice will occur this Tuesday, December 21, at 2338 UTC (6:38 pm EST). This is, of course, when the Earth's Northern Hemisphere has its maximum tilt away from the Sun. It's the shortest day of the year with only 9 hours or so of sunlight (because of the tilt, sunlight doesn't even wrap halfway around the Northern Hemisphere), and the Sun is lowest in the sky (making it cold since it has to heat a larger area).
There's also a full Moon on the solstice (it's full at 0813 UTC or 3:13 am EST). How common is that? Well, first we have to specify a specific time zone. While the full Moon occurs on the solstice day in the contiguous U.S., the point of the full Moon occurs at 10:13 pm on December 20 for Hawaii (the date before the solstice).
Just looking at a December full Moon such that it occurs on the solstice in the UTC time zone (time at longitude 000° which runs through Greenwich, England), it's occurred on December 21, 1980 and December 22, 1999. Since it also occurs on December 21, 2010, you can see it's not evenly spaced (19 years and then 11 years). Next time it occurs will be on December 21, 2094, a gap of 84 years. And, even more interesting, a total lunar eclipse will again occur on that date (but visible in Europe and Asia, not in North America like this one).
So what makes this cycle so complicated? It's because the synodic month, the cycle of lunar phases, is 29.530589 days and the tropical year, length of time from December solstice to December solstice is 365.242740 days. They generally, but not exactly, line up every 19 years (19 * 365.242740 days = 6939.61206 days / 29.530589 days = 234.997 which is damn close to 235. In other words, there are almost, but not quite, exactly 235 synodic months in 19 tropical years. It's called the metonic cycle and, despite being named after the ancient Greek astronomer Meton of Athens, this cycle was known even earlier to the ancient cultures of Mesopotamia.
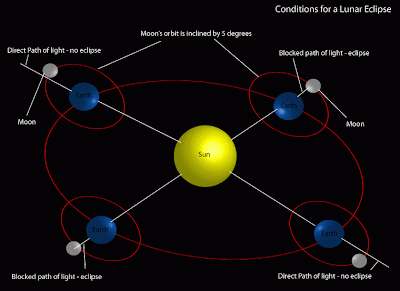
Full Moons on the solstice are not too common, even more uncommon is a total lunar eclipse on the solstice. The last time this happened was December 21, 1638 and the next time will be December 21, 2094. It's truly a once-in-a-lifetime event. Lunar eclipses only occur on the full Moon and only when the Moon lines up with the Sun and Earth. Why not every month? Because the Moon's orbit around the Earth is tilted by about 5° to the plane of the ecliptic. A lunar eclipse only occurs when the Moon is passing up or down through the ecliptic while it's full. This Tuesday morning, three different cycles all line up for us to view.
Of course, and I could have predicted this, the Hudson Valley will be partly cloudy overnight. I'll try to peek out to see what I can see but I'm not optimistic.
There's also a full Moon on the solstice (it's full at 0813 UTC or 3:13 am EST). How common is that? Well, first we have to specify a specific time zone. While the full Moon occurs on the solstice day in the contiguous U.S., the point of the full Moon occurs at 10:13 pm on December 20 for Hawaii (the date before the solstice).
Just looking at a December full Moon such that it occurs on the solstice in the UTC time zone (time at longitude 000° which runs through Greenwich, England), it's occurred on December 21, 1980 and December 22, 1999. Since it also occurs on December 21, 2010, you can see it's not evenly spaced (19 years and then 11 years). Next time it occurs will be on December 21, 2094, a gap of 84 years. And, even more interesting, a total lunar eclipse will again occur on that date (but visible in Europe and Asia, not in North America like this one).
So what makes this cycle so complicated? It's because the synodic month, the cycle of lunar phases, is 29.530589 days and the tropical year, length of time from December solstice to December solstice is 365.242740 days. They generally, but not exactly, line up every 19 years (19 * 365.242740 days = 6939.61206 days / 29.530589 days = 234.997 which is damn close to 235. In other words, there are almost, but not quite, exactly 235 synodic months in 19 tropical years. It's called the metonic cycle and, despite being named after the ancient Greek astronomer Meton of Athens, this cycle was known even earlier to the ancient cultures of Mesopotamia.
Full Moons on the solstice are not too common, even more uncommon is a total lunar eclipse on the solstice. The last time this happened was December 21, 1638 and the next time will be December 21, 2094. It's truly a once-in-a-lifetime event. Lunar eclipses only occur on the full Moon and only when the Moon lines up with the Sun and Earth. Why not every month? Because the Moon's orbit around the Earth is tilted by about 5° to the plane of the ecliptic. A lunar eclipse only occurs when the Moon is passing up or down through the ecliptic while it's full. This Tuesday morning, three different cycles all line up for us to view.
Of course, and I could have predicted this, the Hudson Valley will be partly cloudy overnight. I'll try to peek out to see what I can see but I'm not optimistic.
Friday, December 17, 2010
Men of Salt
Just finished an interesting book - Men of Salt: Crossing the Sahara on the Caravan of White Gold by Michael Benanav (Lyons Press, 2006). Benavav spent 40 days riding camels with a salt caravan across the Sahara from Timbuktu to the salt mines of Taoudenni in the African country of Mali.
by Michael Benanav (Lyons Press, 2006). Benavav spent 40 days riding camels with a salt caravan across the Sahara from Timbuktu to the salt mines of Taoudenni in the African country of Mali.
Armchair travelers, like myself, ofen dream of doing things like this but I have to say as much as I'd love to see the desolate scenery of the Sahara, the reality is I'd hate the experience.
Benavav describes the open sores on his ass from riding a camel, traveling some 20 hours a day (the caravans hurry because if you don't, you'll die out there), the lack of food (one meal a day of rice and, if you're lucky, some dried goat or camel meat), using sand for toilet paper (imagine doing this after having "issues" due to rancid goat meat which the author experienced), drinking nasty water scooped from camel troughs, eating camel offal (guts) as an honored guest.
No thanks, if I ever get the chance to visit (not likely), I'll take the tour that uses a Range Rover and carry freeze-dried meals and toilet paper.
It is a great story though and a gripping read. It's interesting to learn about the men who work during the cooler parts of the year (when the temperature only gets to 120° F) to dig salt which was once as highly valued as gold. Caravans used to travel north from Timbuktu to the Mediterranean with gold and slaves and south with salt from the desert. No more gold on the caravans, slaves are now basically workers trapped in never-ending poverty, but salt is still highly valued and used as a form of currency in this part of Africa.
A salt mine and caravan of salt-laden camels are shown below.
Notice that the layer of white salt (the mineral name for rock salt is halite) has to be reached by digging down a couple of meters through the overlying rock. Imagine doing this on a substandard diet, weeks from civilization, in a remote outpost with no medical care, in 100° F plus temperatures. Not very fun.

Why is there this layer of rock salt in Taoudenni, Mali many hundreds of miles from the ocean? I didn't do any detailed reseach but it appears to be a salt pan, a dry desert lake bed. We know that the Sahara was once more temperate in climate, with grasslands, a few thousand years ago. A lake must have once existed here while it was becoming a desert climate. A lake in a basin with no outlet, losing water only be evaporation, and leaving behind a layer of salt.
Below is a picture of my wife and daughter on the Bonneville salt flats in western Utah near the Nevada border. Same thing happened here. Only difference is that in Mali the salt layer is under another couple of meter of sediments so has to be mined out.

Armchair travelers, like myself, ofen dream of doing things like this but I have to say as much as I'd love to see the desolate scenery of the Sahara, the reality is I'd hate the experience.
Benavav describes the open sores on his ass from riding a camel, traveling some 20 hours a day (the caravans hurry because if you don't, you'll die out there), the lack of food (one meal a day of rice and, if you're lucky, some dried goat or camel meat), using sand for toilet paper (imagine doing this after having "issues" due to rancid goat meat which the author experienced), drinking nasty water scooped from camel troughs, eating camel offal (guts) as an honored guest.
No thanks, if I ever get the chance to visit (not likely), I'll take the tour that uses a Range Rover and carry freeze-dried meals and toilet paper.
It is a great story though and a gripping read. It's interesting to learn about the men who work during the cooler parts of the year (when the temperature only gets to 120° F) to dig salt which was once as highly valued as gold. Caravans used to travel north from Timbuktu to the Mediterranean with gold and slaves and south with salt from the desert. No more gold on the caravans, slaves are now basically workers trapped in never-ending poverty, but salt is still highly valued and used as a form of currency in this part of Africa.
A salt mine and caravan of salt-laden camels are shown below.
Notice that the layer of white salt (the mineral name for rock salt is halite) has to be reached by digging down a couple of meters through the overlying rock. Imagine doing this on a substandard diet, weeks from civilization, in a remote outpost with no medical care, in 100° F plus temperatures. Not very fun.
Below is a picture of my wife and daughter on the Bonneville salt flats in western Utah near the Nevada border. Same thing happened here. Only difference is that in Mali the salt layer is under another couple of meter of sediments so has to be mined out.
While the conditions are hellish, the scenery in Mali can be beautiful. I thought it amusing, however, that the camel drivers laughed at the author when he declared the scenery beautiful - to them it was a terrible place of death.

It's like the early pioneers in the U.S. They believed that chopped down trees and farmed land was beautiful, the wilderness was not. It's hard to appreciate the scenery when you're struggling to survive!
I love reading travel books (not like I can go to many of these places). Anyone have any recommendations of good ones?
Thursday, December 16, 2010
I'm so depressed
Gave a geology final exam today and put an easy question on it (I thought it was a gift to the students). "What is the age of the Earth in Ma?" "Ma" as they all should know, means mega annum or million years and it's how ages are reported on their geologic time scales. Anything between 4,500 and 4,600 Ma would have been accepted for credit but most missed it. The age of the Earth was only mentioned a dozen or more times since the first day of classes (when I mentioned the age of the Earth is one of the main important themes in geology). We also had a 90 minute lecture on radiometric dating and the age of the Earth. I guess no one was listening. Oh, did I mention this was a lab Physical Geology course for science or education majors? Sigh.
Wednesday, December 15, 2010
Orpiment & realgar
Two very pretty, and related, minerals are called orpiment and realgar. Orpiment is a yellow to orange mineral whose name derives from auripigmentum (aurum means "gold" and pigmentum means "pigment" or "color") due to its deep yellow color and use as a paint pigment.
Realgar is a ruby-red mineral whose name comes from the Arabic rahj al-ġār which means "powder of the mine" probably due to its use as a pigment as well.
So what do orpiment and realgar have in common? Turns out they're both arsenic sulfide minerals:
Orpiment = As2S3
Realgar = As4S4
The minerals can also occur together.
Historically orpiment was used as a bright yellow pigment for painting before the development of cadmium yellow. Realgar was used as a ruby-red pigment with one imporant drawback - it fades to a yellow powder after long exposure to light (old paintings where realgar was used now have a yellow color in place of the red the artist intended). Also, one of the major problems with orpiment and realgar pigments is that they're highly toxic, being arsenic compounds.
The fading of realgar pigments also affects the mineral specimens. Realgar needs to be kept in a dark box or drawer, if you leave it out on a bookshelf the mineral will start to break down into a yellow mineral called pararealgar (AsS). I've read that it's the green wavelengths of light that will break down realgar but have no idea why (something I need to research).
Both orpiment and realgar occur in low-temperature hydrothermal veins (fractures in rock in which hot water precipitated minerals), in hot spring deposits, and as sublimates from gasses emitted from volcanic fumaroles. In some areas, these minerals occur with galena (lead sulfide - PbS), gold, and silver deposits. Most of the arsenic produced worldwide comes from these two minerals and another called arsenopyrite (FeAsS).
What use is arsenic? Historically, it was an important poison (it's been called the "Poison of Kings") but today autopsies can detect its presence with some simple chemical tests if it's a suspected cause of death. Victorian women used to ingest arsenic to make them look paler (unlike the tanned commoners who had to work outside). Obviously not a recommended practice. Arsenic was also once used to treat syphilis and other medical conditions. Its poisonous properties have led to its use as a wood preservative (now phased out in the U.S.) and insecticide and fungicide. One high-tech use is in the manufacture of certain semiconductors (notable those made of GaAs - gallium arsenide).
Even though orpiment and realgar are arsenic compounds, they're not really hazardous in a geology lab. Don't chew on the minerals, wash your hands after handling them, and they're fine.
Realgar is a ruby-red mineral whose name comes from the Arabic rahj al-ġār which means "powder of the mine" probably due to its use as a pigment as well.
So what do orpiment and realgar have in common? Turns out they're both arsenic sulfide minerals:
Orpiment = As2S3
Realgar = As4S4
The minerals can also occur together.
Historically orpiment was used as a bright yellow pigment for painting before the development of cadmium yellow. Realgar was used as a ruby-red pigment with one imporant drawback - it fades to a yellow powder after long exposure to light (old paintings where realgar was used now have a yellow color in place of the red the artist intended). Also, one of the major problems with orpiment and realgar pigments is that they're highly toxic, being arsenic compounds.
The fading of realgar pigments also affects the mineral specimens. Realgar needs to be kept in a dark box or drawer, if you leave it out on a bookshelf the mineral will start to break down into a yellow mineral called pararealgar (AsS). I've read that it's the green wavelengths of light that will break down realgar but have no idea why (something I need to research).
Both orpiment and realgar occur in low-temperature hydrothermal veins (fractures in rock in which hot water precipitated minerals), in hot spring deposits, and as sublimates from gasses emitted from volcanic fumaroles. In some areas, these minerals occur with galena (lead sulfide - PbS), gold, and silver deposits. Most of the arsenic produced worldwide comes from these two minerals and another called arsenopyrite (FeAsS).
What use is arsenic? Historically, it was an important poison (it's been called the "Poison of Kings") but today autopsies can detect its presence with some simple chemical tests if it's a suspected cause of death. Victorian women used to ingest arsenic to make them look paler (unlike the tanned commoners who had to work outside). Obviously not a recommended practice. Arsenic was also once used to treat syphilis and other medical conditions. Its poisonous properties have led to its use as a wood preservative (now phased out in the U.S.) and insecticide and fungicide. One high-tech use is in the manufacture of certain semiconductors (notable those made of GaAs - gallium arsenide).
Even though orpiment and realgar are arsenic compounds, they're not really hazardous in a geology lab. Don't chew on the minerals, wash your hands after handling them, and they're fine.
Tuesday, December 14, 2010
Are we depriving our homeschooled children?
My wife and I homeschool our two children. We're pretty open about it even though some people feel the need to share their disapproval with us. This is, of course, something I don't do when talking to other people about how they choose to raise their kids but whatever ("Hey Bill, how come little Courtney dresses like such a tramp?"). Anyway, yesterday someone mentioned something about homsechooling that has now come up at various times from three different people at my job (people who work in higher educations).
It seems that we're depriving our children of the experience of getting the shit kicked out of them by bullies. Evidently this is character-building and teaches kids how to "take care of themselves." I think back to my experience in public school. I was a shy, thin, kid who liked to read and did well on exams. I was made fun of for that by many of my peers. Starting around 4th grade, and extending up until 8th grade, I was not just picked on mercilessly, but also physically beaten up at times. The useless advise from my mother - "Ignore them and they'll leave you alone" is complete bullshit in the Lord of the Flies world of young boys.
It's only when I was about 13 years old that, one day, in the hallway, I slammed one of the bullies into a locker in a violent rage and truly wanted to kill him. I grew large enough, and had endured enough, and finally fought back. Generally I was left alone after that except for the verbal taunts and general ostracism. One of the reasons I dropped out of high school at 14 (16 officially, but stopped attending pretty much in 9th grade). I can truly understand teen suicide from bullying.
You know what? Ever since I left high school I have never, ever been in a physical fight (and I used to hang out in a biker bar years ago). Given my size, and the fact that I regularly lift weights at the gym, I think I could handle myself today but don't feel any macho bullshit reason to do so. Now maybe I'm projecting my bad experiences onto my kids, but I don't think so. My nine-year-old son is much like I was at his age. Shy, a little odd sometimes (in a good way, in my opinion), and he reads more and better than most adults. He would be bullied at school. Maybe he'd learn to handle it, maybe he'd learn to hide the fact that he's smart and likes "nerdy" things, or maybe he'd get bitter and drop out of school like I did.
This doesn't mean we raise our kids in a vacuum, far from it. Between Aikido, history class, Muset orchestra, choir, library programs, Numeracy Club, local nature programs, homeschool co-op and other things we participate in, our kids associate with both homeschooled and public-schooled kids of a variety of ages. Sometimes kids get into arguments and even a shove or hit on occasion although parents quickly step in and defuse the situation. What doesn't occur is mob cruelty and repeated physical and mental abuse of the children.
When I hear of other people's kids crying and telling their mothers they don't want to go to school because they get picked on all day I think we're making the right decision (not just for this, but for a myriad of reasons). I don't think learning to fight is an essential life skill (although they do take Aikido lessons - which is more for self-defense). And, like I said, I've never had to physically fight with anyone, and have not been mercilessly tormented, ever since I left public school. And I don't think it's character-building (at least not in a good way) to be bullied for years of your life.
Perhaps my wife and I should do the following...
It seems that we're depriving our children of the experience of getting the shit kicked out of them by bullies. Evidently this is character-building and teaches kids how to "take care of themselves." I think back to my experience in public school. I was a shy, thin, kid who liked to read and did well on exams. I was made fun of for that by many of my peers. Starting around 4th grade, and extending up until 8th grade, I was not just picked on mercilessly, but also physically beaten up at times. The useless advise from my mother - "Ignore them and they'll leave you alone" is complete bullshit in the Lord of the Flies world of young boys.
It's only when I was about 13 years old that, one day, in the hallway, I slammed one of the bullies into a locker in a violent rage and truly wanted to kill him. I grew large enough, and had endured enough, and finally fought back. Generally I was left alone after that except for the verbal taunts and general ostracism. One of the reasons I dropped out of high school at 14 (16 officially, but stopped attending pretty much in 9th grade). I can truly understand teen suicide from bullying.
You know what? Ever since I left high school I have never, ever been in a physical fight (and I used to hang out in a biker bar years ago). Given my size, and the fact that I regularly lift weights at the gym, I think I could handle myself today but don't feel any macho bullshit reason to do so. Now maybe I'm projecting my bad experiences onto my kids, but I don't think so. My nine-year-old son is much like I was at his age. Shy, a little odd sometimes (in a good way, in my opinion), and he reads more and better than most adults. He would be bullied at school. Maybe he'd learn to handle it, maybe he'd learn to hide the fact that he's smart and likes "nerdy" things, or maybe he'd get bitter and drop out of school like I did.
This doesn't mean we raise our kids in a vacuum, far from it. Between Aikido, history class, Muset orchestra, choir, library programs, Numeracy Club, local nature programs, homeschool co-op and other things we participate in, our kids associate with both homeschooled and public-schooled kids of a variety of ages. Sometimes kids get into arguments and even a shove or hit on occasion although parents quickly step in and defuse the situation. What doesn't occur is mob cruelty and repeated physical and mental abuse of the children.
When I hear of other people's kids crying and telling their mothers they don't want to go to school because they get picked on all day I think we're making the right decision (not just for this, but for a myriad of reasons). I don't think learning to fight is an essential life skill (although they do take Aikido lessons - which is more for self-defense). And, like I said, I've never had to physically fight with anyone, and have not been mercilessly tormented, ever since I left public school. And I don't think it's character-building (at least not in a good way) to be bullied for years of your life.
Perhaps my wife and I should do the following...
Monday, December 13, 2010
Sulfur mines
Phil Plait over at Bad Astronomy brought this to my attention and I can't resist sharing it here. Photographer Olivier Grunewald took stunning images of sulfur miners working in truly hellish conditions in the crater of Kawah Ijen volcano in Java, Indonesia.
Next to a sulfuric acid lake (pH measured at 0.5!) in the caldera are a number of vents emitting volcanic gasses like sulfur dioxide (SO2) and hydrogen sulfide (H2S). These gasses are channeled through ceramic pipes which results in the condensation of liquid sulfur as the gas cools. Deep red molten sulfur pours out of the pipes, cools to the yellow mineral and is then carried out of the area by miners in baskets. The miners work without protective equipment, obviously suffer respiratory problems, and have to hike up and down out of the volcanoes caldera twice a day. Typical miners earn the equivalent of $13 U.S. per day.
Check out the images by Grunewald - they're incredible and look like they were taken on Jupiter's moon Io!
Next to a sulfuric acid lake (pH measured at 0.5!) in the caldera are a number of vents emitting volcanic gasses like sulfur dioxide (SO2) and hydrogen sulfide (H2S). These gasses are channeled through ceramic pipes which results in the condensation of liquid sulfur as the gas cools. Deep red molten sulfur pours out of the pipes, cools to the yellow mineral and is then carried out of the area by miners in baskets. The miners work without protective equipment, obviously suffer respiratory problems, and have to hike up and down out of the volcanoes caldera twice a day. Typical miners earn the equivalent of $13 U.S. per day.
Check out the images by Grunewald - they're incredible and look like they were taken on Jupiter's moon Io!
Sunday, December 12, 2010
GRE scores and majors
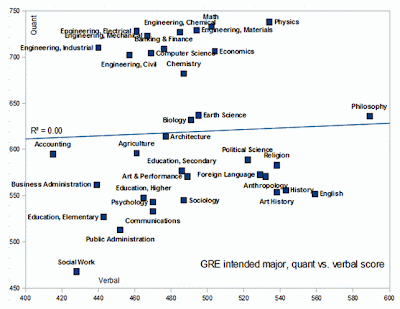
Really interesting plot from Razib Khan's Gene Expressions blog regarding GRE scores and majors (see his blog post for more information and plots). The GREs (Graduate Record Exams) are exams undergraduates take when applying for graduate school (similar to the SATs that high school students take to get into college). Quant refers to the quantitative portions of the GRE (think math and logical reasoning skills) and verbal is self-explanatory. Click plot for a larger image.
Social workers probably can't understand this plot (just kidding!), philosophers are great with language (ever read philosophers? They use their skills for evil), and math and physics majors have great quantitative skills (no surprise there). Anthropologists (blogged about yesterday) and psychologists are not so good at math (art and performance majors outscore them in quant for god's sake - is this why these two fields are filled with so much woo?). Unfortunately, education majors all seem to be below the mean.
Social workers probably can't understand this plot (just kidding!), philosophers are great with language (ever read philosophers? They use their skills for evil), and math and physics majors have great quantitative skills (no surprise there). Anthropologists (blogged about yesterday) and psychologists are not so good at math (art and performance majors outscore them in quant for god's sake - is this why these two fields are filled with so much woo?). Unfortunately, education majors all seem to be below the mean.
Earth science (my field) and biology are middle of the road. Pretty weak in quant when compared to hard sciences like chemistry and physics and those in technical fields like engineering and computer science. Of course these are generalities. Someone majoring in geology but going into geophysics, for example, would have to have great math skills. It's a liability for field geologists, on the other hand, to be very bright (they actually enjoy wearing the same set of underwear for weeks on end and have killed off most of their brain cells with alcohol).
Saturday, December 11, 2010
Anthropologists admit anthropology is not a science
Well, at least some of them, others are not so happy about it.
See this interesting New York Times article "Anthropology a Science? Statement Deepens a Rift."
The problem, and I think it is a problem because I believe most fields of study are best when undergirded by the scientific method, is that many cultural/social anthropologists have ideological agendas and see themselves as advocates rather than objective researchers (although, like in many of the social "sciences", there are anthropologists who argue that the whole concept of scientific inquiry is fundamentally flawed since there's no such thing as an objective researcher or absolute truth).
Here's another article Anthropology Without Science at Inside Higher Ed. Check out this quote from the article:
Still, the change seemed to resonate uncomfortably with some more scientifically oriented anthropologists, who perceived a broader shift in the discipline that they say began decades ago. "It’s become so dominated by, not so much humanistic scholars, but by scholars who are actively hostile" to science, said Raymond Hames, chair of anthropology at the University of Nebraska at Lincoln, and a cultural anthropologist who favors a scientific approach.
Here's another quote discussing a sociocultural anthropology PhD student blogger who:
...argued that continuing to use the term "science" in the association's mission statement had become a concern because it maintained "the colonizing, privileging, superior positionality of anthropology that continues to plague the discipline."
In other words, science is a tool of oppression that old white guys use to beat down indiginous cultures.
The article No Science Please, We're Anthropologists in Psychology Today is also interesting and has a number of links to other reactions in the blogosphere.
This controversy caught me eye because, during my first semester at college, I was an anthropology major since I had an interest in archaeology (still do). I remember the professor in my physical anthropology class saying, in a bitter voice, something like "I hope none of you are anthropology majors, there are absolutely no jobs for you when you graduate." Didn't realize it at the time, but it's quite possible he was an adjunct (part-time) instructor - the slave labor of higher education employment.
Anyway, people are complicated, that's why I like studying rocks - doesn't matter if you're Chinese or American, doesn't matter if you're Christian or atheist, doesn't matter if you're male or female, doesn't matter if you're black or white, a granite is a granite. Sure geologists argue over geological interpretations, but the final arbiter is always hard, physical, DATA and not clever ideological arguments.
See this interesting New York Times article "Anthropology a Science? Statement Deepens a Rift."
The problem, and I think it is a problem because I believe most fields of study are best when undergirded by the scientific method, is that many cultural/social anthropologists have ideological agendas and see themselves as advocates rather than objective researchers (although, like in many of the social "sciences", there are anthropologists who argue that the whole concept of scientific inquiry is fundamentally flawed since there's no such thing as an objective researcher or absolute truth).
Here's another article Anthropology Without Science at Inside Higher Ed. Check out this quote from the article:
Still, the change seemed to resonate uncomfortably with some more scientifically oriented anthropologists, who perceived a broader shift in the discipline that they say began decades ago. "It’s become so dominated by, not so much humanistic scholars, but by scholars who are actively hostile" to science, said Raymond Hames, chair of anthropology at the University of Nebraska at Lincoln, and a cultural anthropologist who favors a scientific approach.
Here's another quote discussing a sociocultural anthropology PhD student blogger who:
...argued that continuing to use the term "science" in the association's mission statement had become a concern because it maintained "the colonizing, privileging, superior positionality of anthropology that continues to plague the discipline."
In other words, science is a tool of oppression that old white guys use to beat down indiginous cultures.
The article No Science Please, We're Anthropologists in Psychology Today is also interesting and has a number of links to other reactions in the blogosphere.
This controversy caught me eye because, during my first semester at college, I was an anthropology major since I had an interest in archaeology (still do). I remember the professor in my physical anthropology class saying, in a bitter voice, something like "I hope none of you are anthropology majors, there are absolutely no jobs for you when you graduate." Didn't realize it at the time, but it's quite possible he was an adjunct (part-time) instructor - the slave labor of higher education employment.
Anyway, people are complicated, that's why I like studying rocks - doesn't matter if you're Chinese or American, doesn't matter if you're Christian or atheist, doesn't matter if you're male or female, doesn't matter if you're black or white, a granite is a granite. Sure geologists argue over geological interpretations, but the final arbiter is always hard, physical, DATA and not clever ideological arguments.
Friday, December 10, 2010
Lithium
Lithium's an interesting element. It's one of the few elements that formed along with hydrogen during the Big Bang at the origin of the universe and is number 3 on the periodic table. It's one of the alkali metals found in Group I (the first column) of the table. These are silvery, soft, low density metals that have one lone electron in their outermost shell and are highly reactive with water (they are generally stored in mineral oil or kerosene).


Mix lithium with water and you get a highly energetic reaction:
Take lithium hydroxide (LiOH) and mix it with tallow (animal fat) and you get lithium stearate (LiC18H35O2), a component of grease (ever greased wheel bearings?).
Lithium hydroxide is also useful in spacecraft, submarines, and scuba rebreathers where it helps scrub carbon dioxide (CO2) from exhaled air.

A huge use of lithium is for batteries. Most lithium batteries use lithium for the anode (negative end) and manganese dioxide (MnO2) for the cathode (positive end) of the battery. Lithium batteries are used in applications that require relatively low current (but can deliver high current pulses) and a long-life. In other words, a lot of consumer electronics.
Lithium, being so reactive, is never found as a metal by itself naturally, it's mined from ore minerals like spodumene - LiAl(SiO3)2 - a proxene group mineral. Spodumene forms in lithium-rich granite pegmatites - igneous rocks which form from cooling magma at depth and grow relatively large crystals due to the presence of water and other volatiles dissolved in the molten rock.
 Single crystals of spodumene as long as 50 feet have been reported from the famous, but now defunct, Etta Mine in the Black Hills of South Dakota. Below is a cross-section of the mine showing the spodumene crystals in the central ore body, an historic photo of a 47 meter long spodumene "log" excavated in 1904, and a photo I took of a tunnel at the Etta Mine a few years ago.
Single crystals of spodumene as long as 50 feet have been reported from the famous, but now defunct, Etta Mine in the Black Hills of South Dakota. Below is a cross-section of the mine showing the spodumene crystals in the central ore body, an historic photo of a 47 meter long spodumene "log" excavated in 1904, and a photo I took of a tunnel at the Etta Mine a few years ago.



Mix lithium with water and you get a highly energetic reaction:
2 Li (s) + 2 H2O (l) → 2 LiOH (aq) + H2 (g)
Take lithium hydroxide (LiOH) and mix it with tallow (animal fat) and you get lithium stearate (LiC18H35O2), a component of grease (ever greased wheel bearings?).
Lithium hydroxide is also useful in spacecraft, submarines, and scuba rebreathers where it helps scrub carbon dioxide (CO2) from exhaled air.
2LiOH + CO2 → Li2CO3 + H2O
Astronaut checking lithium hydroxide canisters on the space shuttle Endeavour (STS-126)
The product of this reaction is lithium carbonate (Li2CO3), a very useful industrial chemical used in glassmaking for bakeware, in ceramic glazes, as an ingredient in cement to help it set faster, in tile adhesive, and other applications.
Lithium carbonate is also used as a medication. It turns out that that it acts as a mood stabilizer in bipolar disorders. It's not well-understood why it works (but certainly shows how chemistry affects our moods). In 1929, lithium citrate (Li3C6H5O7), also used as a mood stabilizer, was placed into a soda originally called "lithiated lemon-lime soda." Today we know it as 7-Up (no lithium in it anymore!).
A huge use of lithium is for batteries. Most lithium batteries use lithium for the anode (negative end) and manganese dioxide (MnO2) for the cathode (positive end) of the battery. Lithium batteries are used in applications that require relatively low current (but can deliver high current pulses) and a long-life. In other words, a lot of consumer electronics.
I've read that lithium scavenged from old batteries can be used in the production of methamphetamine. This has led to completely ridiculous restrictions (like those for Sudafed) in some localities restricting the amount of batteries you can purchase at one time.
Spodumene has two gem varieties. One is pale, emerald green hiddenite first found in North Carolina by eploration geologist William Earl Hidden. Hidden was actually searching for sources of platinum in the area for Thomas Edison in 1879 (which was a failure). Specimen below is from Pakistan.
The second variety of spodumene of note is kunzite, a pink to pale purple variety. Kunzite has a tendency to fade in bright light leading to its nickname of "evening stone" when used as jewelry. First found in California in 1902, it's named after the famous mineralogist George F. Kunz who worked for both the American Museum of Natural History and the Tiffany Company among other things. Below is some kunzite from Pala, California.
Another mineral containing lithium is a variety of Elbaite tourmaline (named for deposits in Elba, Italy) called "watermelon tourmaline" for its zoned red/green color. Elbaite has the horrendously complex formula Na(Li,Al)3Al6Si6O18(BO3)3(OH)4.
Tourmalines (the name denotes a whole group of minerals) are also found in granite pegmatites and often occur in association with spodumene. The sample above is from the famous Plumbago Mountain deposit in Maine.
Like I said, it's an interesting element.
Thursday, December 9, 2010
Why do you homeschool?
People sometimes ask me why I homeschool. Could be due to seeing results like the one below comparing the U.S. to other developed (and not so developed) countries around the world.
Maybe I should start teaching my kids Chinese so they'll prove useful to our Asian overlords in another decade or two.
Could also be due to fact that the majority of our students require remedial English and math (sometimes they require READING courses) even though they're local high school graduates.
There's something horribly wrong with education in the U.S. We spend something approaching $20,000/year/student for our local high school and some of the students who graduate are functionally illiterate. The public should expect better results for that amount of money spent.
Many of these recent graduates can't read or write well enough to take Freshman English 101 at a community college (they can't write a coherent paragraph on a placement exam). They can't place into something we call College Algebra (a course which is indistinguishable to what I took as algebra in the 9th grade 30+ years ago). Yet they have NYS Regent's diplomas. What the fuck? I think "No child left behind" is better stated as "No child too damn ignorant to graduate".
Maybe I should start teaching my kids Chinese so they'll prove useful to our Asian overlords in another decade or two.
Wednesday, December 8, 2010
Liberal Arts
A response to students who complain about all the different courses they have to take in college...
The liberal arts are not merely indispensable; they are unavoidable. Nobody can decide for himself whether he is going to be a human being. The only question open to him is whether he will be an ignorant, undeveloped one or one who has sought to reach the highest point he is capable of attaining. The question, in short, is whether he will be a poor liberal artist or a good one.
Robert Maynard Hutchins, Great Books of the Western World. Volume 1: The Great Conversation: The Substance of a Liberal Education (1952)
The liberal arts are not merely indispensable; they are unavoidable. Nobody can decide for himself whether he is going to be a human being. The only question open to him is whether he will be an ignorant, undeveloped one or one who has sought to reach the highest point he is capable of attaining. The question, in short, is whether he will be a poor liberal artist or a good one.
Robert Maynard Hutchins, Great Books of the Western World. Volume 1: The Great Conversation: The Substance of a Liberal Education (1952)
NASA scientists eviscerated
Great analysis of the Science paper and NASA press conference last Thursday (see post 1 and post 2) by Dr. Rosemary (Rosie) Redfield who runs a microbiology lab at the University of British Columbia.
Read it here
A bit technical but well worth reading if you're interested in NASA's claims about these supposedly arsenic-based bacteria. A couple of great quotes:
"Bottom line: Lots of flim-flam, but very little reliable information... If this data was presented by a PhD student at their committee meeting, I'd send them back to the bench to do more cleanup and controls."
and
"I don't know whether the authors are just bad scientists or whether they're unscrupulously pushing NASA's 'There's life in outer space!' agenda. I hesitate to blame the reviewers, as their objections are likely to have been overruled by Science's editors in their eagerness to score such a high-impact publication."
Love it - this is how science works! Put your idea out there, give other people the chance to criticize it, and if it's a good idea supported by evidence it becomes accepted science. If not, which I think is the case here, it dies.
What's unfortunate is that our tax dollars are going to support bad science by people more interested in grabbing headlines.
Read it here
A bit technical but well worth reading if you're interested in NASA's claims about these supposedly arsenic-based bacteria. A couple of great quotes:
"Bottom line: Lots of flim-flam, but very little reliable information... If this data was presented by a PhD student at their committee meeting, I'd send them back to the bench to do more cleanup and controls."
and
"I don't know whether the authors are just bad scientists or whether they're unscrupulously pushing NASA's 'There's life in outer space!' agenda. I hesitate to blame the reviewers, as their objections are likely to have been overruled by Science's editors in their eagerness to score such a high-impact publication."
Love it - this is how science works! Put your idea out there, give other people the chance to criticize it, and if it's a good idea supported by evidence it becomes accepted science. If not, which I think is the case here, it dies.
What's unfortunate is that our tax dollars are going to support bad science by people more interested in grabbing headlines.
Tuesday, December 7, 2010
Doll's eyes
A botanical picture from last summer I found while looking through my stored images. It's a neat plant called doll's eyes or white baneberry (Actaea pachypoda). The specific name pachypoda ("big foot") refers to its thick underground rhizome.
It was growing in the Mohonk Preserve near Spring Farm. The berries are poisonous - they're cardiogenic, interfere with the function of the heart, leading to cardiac arrest and death (birds eat them without ill effect, however). The name "white baneberry" should provide a clue that the berries aren't too good!
Supposedly Native Americans made a medicinal tea from the roots of this plant to relieve pain after childbirth - the roots have the active ingredient of β-sitosterol glucoside which reduces menstrual cramping. This preparation is sometimes called white cohosh since it's similar to black cohosh (from the roots of the related plant Actaea racemosa) sold as an herbal supplement used to relieve vasomotor symptoms of menopause.
I wouldn't recommend use of this plant, however, since the entire plant is considered poisonous (most medicines are, after all, lower dosages of chemicals that will kill you - the problem is getting a correct dosage with something like tea from a plant root!).
I just thought it was a neat-looking plant that I had never noticed prior to this past summer.
It was growing in the Mohonk Preserve near Spring Farm. The berries are poisonous - they're cardiogenic, interfere with the function of the heart, leading to cardiac arrest and death (birds eat them without ill effect, however). The name "white baneberry" should provide a clue that the berries aren't too good!
Supposedly Native Americans made a medicinal tea from the roots of this plant to relieve pain after childbirth - the roots have the active ingredient of β-sitosterol glucoside which reduces menstrual cramping. This preparation is sometimes called white cohosh since it's similar to black cohosh (from the roots of the related plant Actaea racemosa) sold as an herbal supplement used to relieve vasomotor symptoms of menopause.
I wouldn't recommend use of this plant, however, since the entire plant is considered poisonous (most medicines are, after all, lower dosages of chemicals that will kill you - the problem is getting a correct dosage with something like tea from a plant root!).
I just thought it was a neat-looking plant that I had never noticed prior to this past summer.
Monday, December 6, 2010
Solar filament eruption
Over the next few months, we're moving into an active phase of the Sun's 11-year cycle. There have been some big sunspots visible lately - this picture is from around noon EST today:
There's also been a huge solar filament stretching around the Sun's southeastern limb (the image above is upside down since that's the way it's seen through the telescope so the filament is actually associated with that large sunspot visible in the picture).
The filament was 700,000 kilometers in length (that's about the radius of the Sun!) and erupted today. Most of the energy was directed away from the Earth so shouldn't result in any auroras but here's some spectacular footage from NASA's Solar Dynamics Observatory. Click on the image to view as a movie.
There's also been a huge solar filament stretching around the Sun's southeastern limb (the image above is upside down since that's the way it's seen through the telescope so the filament is actually associated with that large sunspot visible in the picture).
The filament was 700,000 kilometers in length (that's about the radius of the Sun!) and erupted today. Most of the energy was directed away from the Earth so shouldn't result in any auroras but here's some spectacular footage from NASA's Solar Dynamics Observatory. Click on the image to view as a movie.
Our Sun is an active place!
Sunday, December 5, 2010
Aluminum oxide
As a metal, it has a myriad of uses and is notable for being resistant to corrosion. Discovered in 1825, but difficult to extract from its mineral ore, aluminum was as highly valued as gold or silver for a time due to its shiny, light-weight, corrosion-resistent properties.
In the mid-1800s, the French Emperor Napolean III served his most distinguished guests on aluminum plates rather than gold ones since aluminum was the rarer metal. In 1884, a six-pound pyramid of aluminum was placed atop the Washington Monument (picture at left). It wasn't until around 1890 that industrial processes were developed which allowed aluminum to be refined more easily and it become a more pedestrian metal we encounter on a daily basis.
When iron (Fe) is exposed to air, iron oxide (Fe2O3) rust forms. The soft rust flakes off exposing more iron to air forming more rust and the iron eventually corrodes away. Aluminum, however, is a different story. When metallic aluminum is exposed to air, it almost instantly forms a coating of aluminum oxide (Al2O3) a few atoms thick. This substance is incredibly hard (the only thing harder are diamonds) and result in aluminum being so resistant to corrosion.
Aluminum oxide (Al2O3) also forms naturally as a mineral called corundum.
South of Peeksill, NY, in the mid-Hudson Valley, is a deposit of emery formed 430 million years ago (Silurian Period) when blocks of the Manhattan Formation schist (the same rock exposed in Central Park in Manhattan) were metamorphically altered by intruding magma forming what's called the Cortlandt Complex. This altered rock is called emery and composed of corundum mixed with some other minerals species and mined for use an an abrasive (women who do their nails know what emery boards are). There aren't many emery mines around, one of the biggest is on the Greek island of Naxos.
Throw a little chromium (replace Al3+ with a few Cr3+) in corundum as an impurity and you have a variety known as ruby.
Rubies are relatively rare with the best historically coming from Burma (Myanmar). The word corundum, by the way, comes from the Tamil (a language in India) word kuruntam meaning ruby.
Sapphires, which come in a variety of colors are another form of corundum due to impurities substituting for the aluminum ions. If iron (Fe2+) and titanium (Ti4+) substitute, a deep blue color results.
Simple aluminum oxide... Corrosion protection for aluminum metal, abrasive material we've all used in sandpaper and emery boards, and fantastic gemstones like rubies and sapphires.
Subscribe to:
Posts (Atom)